Exclusive Deals
Best Prices
Best Products
Fast Delivery
Exclusive Deals
Best Prices
Best Products
Fast Delivery
Orobis ImmunoTM Nanoadjuvant technology utilizes quantum technology and silk protein for enhanced immunity & prolong protection.
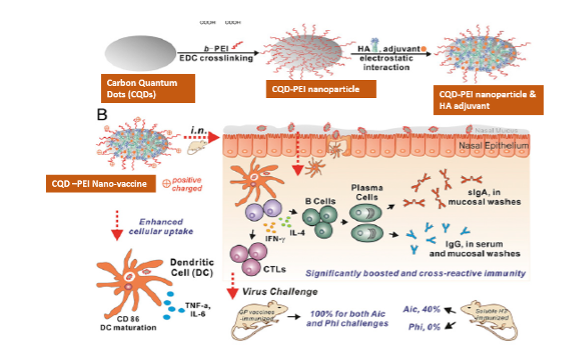
With a greater understanding of the mechanisms by which the innate immune response controls the antigen-specific response, the adjuvants’ action mechanisms are explored to attain desirable enhanced and prolonged immunity. We suggest a nanoadjuvant for improved and sustained antigen delivery to antigen-presenting cells using nanotechnology. PEI-charged carbon quantum dots have provided superior antigen delivery to tissue-specific dendritic cells and macrophages. Fibroin-based nanoparticles made from silk worms deliver antigen continuously, promoting immunity. It has been suggested that the use of CQD-PEI and Fibroin nanoparticles together can lead to both maximal immune response and long-lasting protection against particular bacterial and viral antigens.
When given with vaccine antigens, adjuvants are any number of substances that increase the immunogenicity of the vaccination. The use of current vaccinations is progressively revealing its drawbacks, including the incapacity to produce long-term protective immunity, the weakness of immunity in some flocks for a variety of reasons, and the incapacity to produce efficient cellular immunity. Adjuvants fall into two categories: immunostimulants and delivery mechanisms. Immunostimulants are signal molecules that lead to the maturation and activation of APCs by targeting specific receptors on APCs cells. Specifically, immunostimulants act as PAMPs, DAMPs or their mimics, which can interact with PRRs on APCs to trigger an innate immune response and lead to the activation and maturation of APCs. Mature APCs terminate their phagocytic antigen activity and enhance their ability to present antigens and express high levels of co-stimulatory signals and cytokines. Effective adjuvants provide the antigen presentation signals are the antigen peptides-major histocompatibility complexes (MHC) that are presented on the surface of APCs after antigens have been taken up and processed. Co-stimulatory signals include co-stimulatory molecules (e.g., CD40, CD80, CD86) expressed on the surface of APCs and secreted inflammatory cytokines (e.g., IL-6, IL-10, IL-12, and TNF-α). The production of these two signals can strongly induce the activation of naive T cells, leading to an enhanced adaptive immune response.
No matter which type of antigen is given, the body breaks down the ingredients or they’re destroyed by the immune system. It is a great challenge when vaccine efficacy requires high serum antibody titers, combined with long-lived antibody responses. Vaccines are typically administered and require multi-dose series of injections to induce an adequate level of protection. This means vaccines can’t cause long-lasting health effects and provide varied vaccine responses and require booster dose to sustain antibodies and cellular immunity. To achieve sustenance of immunity for longer period of time, it is required to deliver antigen to achieve prolonging antigens bioavailability by (1) allowing a sustained release of antigens, (2) forming immune niches, and (3) providing cargo protection for antigens. The prolonged antigens bioavailability will ensure that there are sufficient antigens available to the APCs. Accordingly, this will lead to more MHC-antigen peptides signal production.
Intranasal vaccination with recombinant protein/peptide-based vaccines is an attractive strategy with high safety. Purified protein/peptide antigens eliminate safety concerns as allergies, possess minimal side effects, and enable quick and cost-effective production. However, soluble protein vaccines are poorly immunogenic by i.n. immunization due to the harsh and tolerogenic mucosal environment. The selection of appropriate formulations and adjuvants is crucial for successful i.n. vaccines. The vaccination specific route as intranasal can induce both systemic and mucosal immune responses. Secretory immunoglobulin A (sIgA) and immunoglobulin G (IgG) may prevent viral infection at the portal of virus entry. The viral mucosal immunity has been reported to confer cross protection against heterologous and heterosubtypic viruses. Intranasal (i.n.) immunization is a promising vaccination route for infectious respiratory diseases such as influenza. Recombinant protein vaccines can overcome the safety concerns and long production phase of virus-based influenza vaccines.
Nanoparticles (NPs) as antigen delivery vehicles known as Nanoadjuvant via carrying the antigen to Antigen Presenting Cells (APCs) such as macrophages and dendritic cells (DCs) could improve the immunogenicity of vaccines. The application of Nanoadjuvant in vaccines will be a powerful tool due to its strong immunological characteristics along with the appropriate technical and functional characteristics. Nanocarriers based delivery systems can extend shelf life of the vaccines from premature degradation, enhance stability, had good adjuvant properties, and also control the release of antigen. In addition, the particle nature of nanoparticles provides a better antigen presentation to the APCs. Nanocarriers based delivery systems provide an appropriate route of administration for antigen molecules and increase cellular uptake, resulting in strong innate, humoral, cellular, and mucosal immune responses compared to other antigen delivery systems. Delivering NPs coated antigen to local tissue would generate TRM cells response for strong and sustainable immune response.
Polyethylenimine (PEI) is one of the well-known cationic polymers. Increasing evidence has shown that PEI could act an important role as adjuvants in nanovaccines. The effects of neat PEI itself and PEI-based nanoparticles (NPs) in antigen uptake and presentation, which is the foundation for understanding the interplay between PEI and APCs. However, soluble protein vaccines are poorly immunogenic if administered by an i.n. route. A recent study found that polyethyleneimine (PEI) has potent mucosal adjuvant activity for viral subunit soluble glycoprotein antigens. PEI-based nanovaccine can promote antigen presentation to mature B cells in GC, and facilitate differentiation of B cells into long lived plasma cells that populate the bone marrow. NP not only can induce faster antibody titer but also can cause long-term antibodies in vivo.
Nanoparticle-based vaccine combinations especially encapsulated form can provide protection in a long time period. Carbon Quantum Dots (CQDs) is a small size carbon nanoparticle which can be charged with PEI for holding antigen at higher amount. Being an inert nature carbon, CQDs will always be non-reactive in the body and can perform delivery vehicle for various molecules. We speculate that intranasal immunization with CQD-PEI combined with antigens could improve mucosal and systemic immunity. A noninvasive intranasal (i.n.) vaccine can induce mucosal immune responses in respiratory tracts, preventing infection at the portal of virus entry. However, the absence of appropriate mucosal adjuvants at present hinders the development of such a vaccine. Here, we have developed polyethyleneimine functionalized two-dimensional nanoparticles (CQD-PEI) that will show high antigen-loading capacities and superior immunoenhancing properties. Robust and broadly reactive immune responses will induce with i.n. immunization with CQD-PEI nanoparticles, conferring protection against homologous and heterologous viruses. With versatility and flexibility, nanoparticles can be easily adapted for constructing mucosal vaccines of different respiratory pathogens.
It would be advantageous to develop vaccines that induce protective immunity with fewer doses, ideally just one. Single-dose vaccines would be ideal to maximize vaccination coverage, help stakeholders to greatly reduce the costs associated with vaccination. Tissue-resident memory T (TRM) cells are critical mediators of immunity against infections in various tissues. Typically, TRM cells are located at barrier surfaces and, therefore, occupy the initial sites of infection and provide rapid protection. The intraepithelial CD4+TRM cells are in a favourable position to recognize the pathogen and initiate a protective response. During infection or inflammation, myeloid-lineage cells, including monocytes and neutrophils, infiltrate in local tissues and then secrete cytokines and chemokines, which induce entry of activated effector T cells into inflamed tissues. Resident macrophages secrete CCL5 for CCR5+CD4+T cells, which sustain CD4+TRM cells in the mucosa. The local CXCL10 and CXCL9 have been shown to facilitate entry of CXCR3+CD8+T cells into the epithelium. In the context of infection or immunization, epithelial cells are capable of acting as antigen-presenting cells by secreting cytokines and chemokines, and subsequently recruit circuiting immune cells to initiate the local immune response. Typically, TRM cells are located at barrier surfaces and, therefore, occupy the initial sites of infection and provide rapid and prolonged protection.
The unique properties of protein-based NPs such as proper biodegradable and biocompatibility over synthetic polymers make growing attention in developing protein-based NPs vaccine delivery systems. Silk fibroin (SF) is a protein-based biomacromolecule derived from Bombyx mori. SF has been extensively used in biomedical fields as a biomaterial in the form of films, three dimensional scaffolds, hydrogels, electro-spun fibers, micro, and nanospheres. Tuneable biodegradability and biocompatibility properties, low toxicity, appropriate mechanical features and functionality, and providing sustained release introduce silk fibroin nanoparticles (SFNPs) as an appropriate vaccine delivery system. The application of SFNPs as adjuvant along with immune-stimulating agent can be used as nanoadjuvant or carrier vehicle for controlled release of antigen.
Antigen release from SFNPs can be delayed with different concentration of SFNPs. SFNPs in the vaccine formulations promoted humoral and cellular (IFN-γ, IL-4, and IL-17) immune responses in comparison to controls. Immunization of mice with antigen-encapsulated SFNPs significantly increased the total IgG as well as IgG2a/IgG1 ratio. In addition, this formulation triggered concurrently type1 (Th1) and type2 (Th2) immune responses, with a Th1-polarized response. Higher antibody response by encapsulated antigen in SFNPs as compared to the antigen formulations alone, suggests a strong adjuvant activity of SFNPs and their capability in promoting DC maturation as well as enhancing antigen uptake and presentation.
We aimed to fabricate unique combination of PEI doped Carbon Quantum Dots (CQDs) for antigen loading and SFNPs as sustained release of antigen for a designed vaccine candidate. The dual system of CQD-PEI and SFNPs will provide antigen delivery to resident APCs effectively and slow release of antigen at the site will give sustainable humoral and cellular immunity for longer time. Being a receptive nature of CQD-PEI would provide effective antigen loading and delivering to macrophages or dendritic cells due to its nanostructure. SFNPs are made of inert protein will provide sustain release without its reactivity to the body. The novel Nanoadjuvant is biodegradable and leave no toxicity in a long run.
